Understanding Water Quality Indicators

Quick Links
LSM Programs & Tools
Latest News from LSM
Forms & Data Sheets
Transparency, Polluted Stormwater Runnoff, and Algae
There are many imminent threats to Maine lakes. Near the top of the list, and perhaps the most pervasive, is the potential for lakes to become nutrient enriched and more biologically productive due to development in lake watersheds. This condition is often characterized by declining water clarity (transparency), resulting from an increase in the growth of algae.
Excess algae in lake water can cause a disturbance to the normal equilibrium of the aquatic ecosystem. As these high populations of algae die, they are decomposed by bacteria in the process consuming dissolved oxygen gas in the water. Increased algal growth can lead to a decline in oxygen levels, especially during the warm summer months. Similar to the reason why most fish tanks need an aerator or waterfall filter, the loss of oxygen reduces critical habitat for cold-water fish (notably trout and salmon), and it can accelerate the decline of water quality.
The enrichment of lakes with the nutrient phosphorus and excess algae, resulting from watershed development, is referred to as “cultural eutrophication”. Stormwater runoff from disturbed or developed areas of lake watersheds typically carry high concentrations of phosphorus, sediment particles, and other pollutants into lakes. Lake watershed boundaries may be situated close to the shoreline, or they may extend for miles away from the lake. In either case, stormwater runoff from developed areas of lake watersheds is a potential threat to water quality, unless conservation practices are in place for controlling stormwater runoff. Information about these conservation practices can be found in the Watershed Assessment program.
For this reason, the primary focus of volunteer water quality monitoring is the collection of information about the lake over time. Water quality data gathered by volunteers can be used to determine whether individual lakes are experiencing CE to a greater, lesser, or stable extent. Many years of data are generally required to make these determinations with confidence. To learn more about becoming a volunteer water quality monitor, visit our Water Quality Certification page.
Many formats for monitoring water quality by LSM volunteers exist but most fall into one of these categories:
- Secchi Disk Transparency (Lake Water Clarity) & Total Phosphorus Surface Concentration Monitoring
- Dissolved Oxygen and Thermal Profile Monitoring
- Advanced Monitoring of Physical, Chemical, and Biological Parameters
In order to ensure the highest possible quality assurance with volunteer collected data, all the forms of sampling require an initial certification, and recertification on a regular interval.
Dissolved Oxygen, Thermal Stratification, and Henry’s law
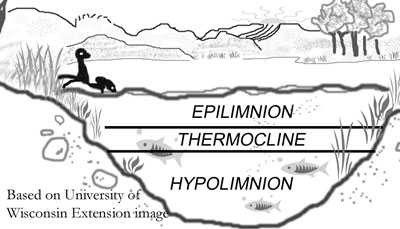
As lake water is warmed in the summer the water in deeper lakes forms three distinct temperature layers:
- warmer (less dense) epilimnion layer at the surface
- the thin thermocline (transition) layer
- the cold and deep hypolimnion layer
Biological activity peaks in lakes and ponds during the warm weather months. It is also at this time that the phenomenon of thermal stratification occurs. The combined influence of the two processes has a pronounced effect on water chemistry, and in particular on dissolved oxygen levels. The physical isolation of deep, cold water at the bottom of a lake from the surface water during summer stratification prevents the oxygen supply in the deeper water from being replenished. The period of isolation varies from one body of water to another, and depends on depth, and the influences of weather. Stratification can occur to varying extents and last from days to weeks to months. For some shallow waterbodies, it may exist for even shorter periods of time during warm, calm periods, but is disturbed when winds become stronger. For these shallower waterbodies, chronic stress from stratification is more of a threat, than sustained periods of oxygen depletion.
Some oxygen loss occurs naturally during the summer months as water temperatures rise. This is due to an inverse relationship between dissolved gasses in a liquid and the temperature of the liquid; also known as Henry’s law. In other words, cold water is able to contain more dissolved oxygen than warm water (all other factors being held equal). You may have witnessed this law in action when you open a cold versus a warm bottle or can of soda. More gas escapes and the beverage is less bubbly when warm as compared to when it it cold.
As lakes become more biologically productive, and organic matter accumulates, the potential for oxygen levels to decline increases. With organic matter decomposition in the deep, stratified areas, that have warmed to some extent, the threat of oxygen depletion increases. Oxygen depletion, or even just depression, stresses many species of fish and other aquatic biota, and under certain circumstances (such as large amounts of ferric phosphate sediments), it can cause an acceleration in the decline of water quality.
The Impact of Nutrients
Total Phosphorus Long-term Data Distribution
Because most lakes in Maine have significant concentrations of nitrogen, phosphorus acts as the most limited for algae growth. An increase in the concentration of total phosphorus in lake water generally indicates a potential increase in biological productivity (trophic state) of the system. Tracking in-lake phosphorus levels over time can forewarn of emerging issues and provide evidence of the cause of degradation of lake health. When combined with Secchi transparency readings and DO / thermal profiles, total phosphorus (TP) samples provide additional information about lake ecosystem dynamics and help in understanding how to improve the lakes water quality. However, as is the case with most indicators of lake water quality, the concentration of TP varies within individual seasons, and from one year to the next.
Learn more about other indicators and their distribution among Maine Lakes:
- Total Phosphorus Long-term Distribution
- Chlorophyll a Long-term Distribution
- Color Long-term Distribution
- Conductivity Long-term Distribution
- pH Long-term Distribution
- Total Alkalinity Long-term Distribution
